Electronics:A first Course
Introduction to Electronics
In this fast developing society, electronics has come to stay as the most important branch of engineering. Electronic devices are commonly used in a large number of applications that formerly relied on mechanical or electric systems for their operation. Examples are electronic controls in automatic cameras, electronic ignition systems in cars, and electronic control in domestic equipment, such as washing machines.
The branch of engineering which deals with current conduction through a vacuum or gas or semiconductor is known as electronics.Electronics essentially deals with electronic devices and their utilization. It handles electric circuits containing passive elements (Resistors, Capacitors etc..), active elements (Diodes, Transistors, LEDs etc..), and other underlying techniques making it as an important part of engineering.

Role of Electronics:
Electronics has an all-important role in a country's development process today. Electronics plays a catalytic role in enhancing production and productivity in key sectors of the economy, whether it relates to infrastructure, process industries, communication, or even manpower training. High-tech areas today depend heavily on electronics.
Electronics is conventionally classified into consumer, industrial, defence, communications and information processing sectors. In recent times, medical electronics, and systems for transportation and power utilities have become important segments on their own.
Applications of Electronics:
Electronics is widely used in Consumer Electronics, Automotive, telecommunication, Healthcare, Aerospace and Robotics.
Matter to Electrons
Matter:
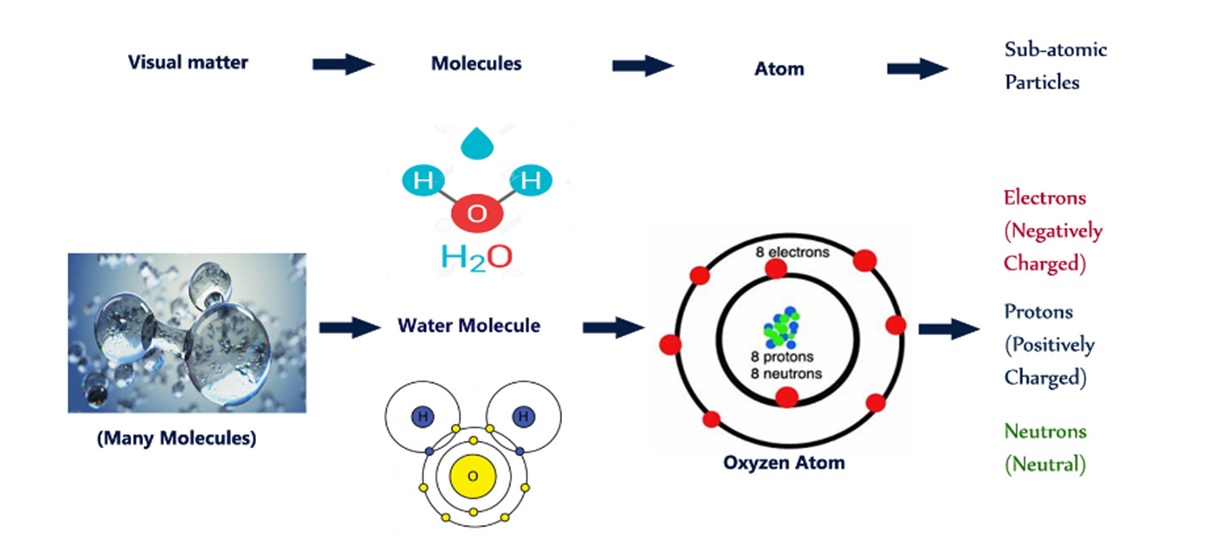
Everything in the world is made of matter. Matter is anything that has mass (weight) and occupies space. Matter can be made up of a group or series of different atoms to form a molecule. Matter has three states: Solid, Liquid, and Vapor.
Molecule:
All matter is made of molecules, or combinations of atoms, that are bound together to produce a given substance, such as water or salt or glass. If you could keep dividing water, for example, into smaller and smaller drops, you would eventually arrive at the smallest particle that was still water. That particle is a molecule, which is defined as the smallest bit of a substance that retains the characteristics of that sub-stance.
The molecule of water is known in chemical notation as H2O. That means the molecule is actually made up of two atoms of the element hydrogen (H) and one atom of the element oxygen (O). These atoms, themselves, are not water but the separate elements of which the molecule of water is composed.
Molecules are made up of atoms, which are bound together to produce a given substance.
Atom:
One of the basic building blocks in the universe for matter is the atom. All matter - gas, liquid, or solid - is made up of molecules or atoms joined together. These atoms are the smallest particle into which an element or substance can be divided without losing its property.
Atomic Particles:
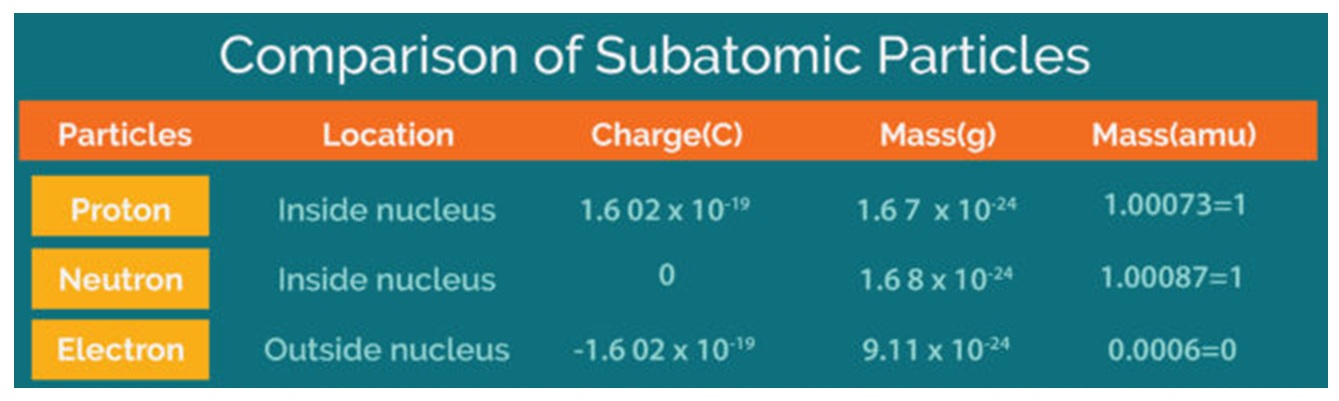
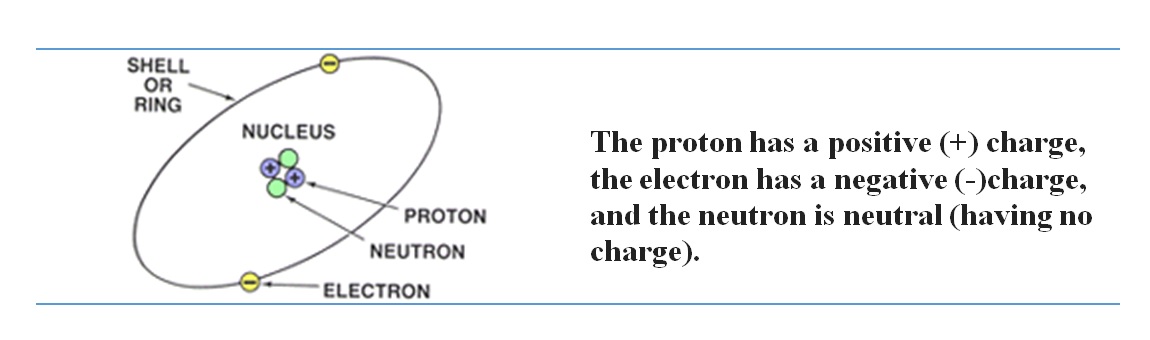
Atoms are made of even smaller particles, called protons, neutrons, and electrons. These particles differ in weight (the proton is much heavier than the electron) and charge. The weights of the particles need not concern you, but the charge is extremely important in electricity. The proton has a positive (+) charge, the electron has a negative (-)charge, and the neutron is neutral.
Protons and neutrons form an atom's nucleus (its center), and electrons orbit the nucleus like planets orbiting the sun. The electrons move around the nucleus of the atom in various orbits. Such electrons are not free: They are locked into the atom because they are attracted by the nucleus.
Balanced Atom:
Atoms normally have an equal number of electrons and protons. Atoms have no electrical charge. They are neither positive nor negative. They are electrically neutral or BALANCED. The negative charge of the electrons will cancel the positive charge of the protons, thus balancing the charge of the atom. This cancellation of charges creates a natural attraction or bonding between the positive proton and the negative electron.
In a stable or balanced atom the orbiting electrons remain in their orbits as long as nothing upsets the balance.When something upsets this balance, then some of the electrons become "knocked" out of their orbits.
This unbalanced condition can be caused by rubbing cat's fur on amber, passing a wire through a magnetic field, or putting two chemicals together, as in a dry cell battery.
Ion Particles:
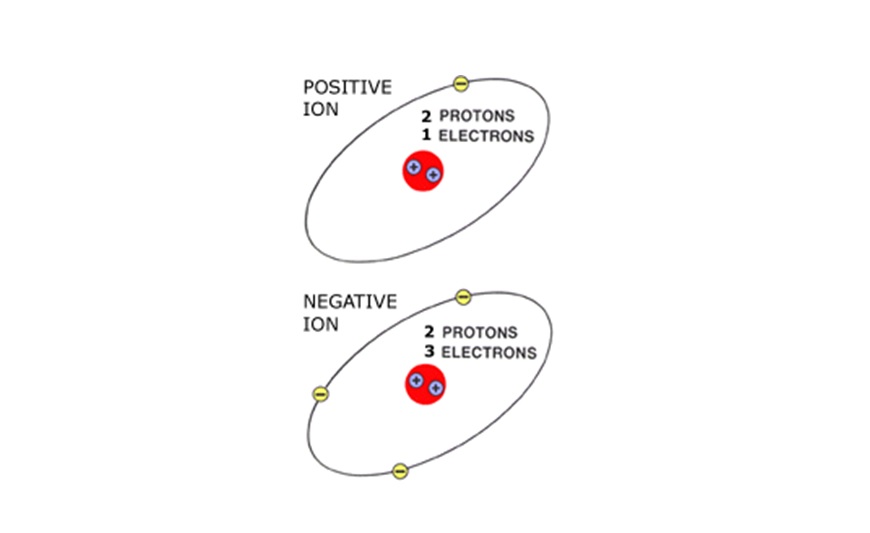
When an atom loses or gains an electron, an imbalance occurs. The atom becomes either a positively or negatively charged particle called an ION.These unbalanced charged ION particles are responsible for electron flow (electricity). IONs will take or release an electron to become balanced again.
A positive (+) ION has one less electron than it has protons. A negative (-) ION has one more electron than it has protons. The positive ION attracts a negative ION to become balanced. This attraction or difference in electrical potential causes electron flow.
Electron Orbits:
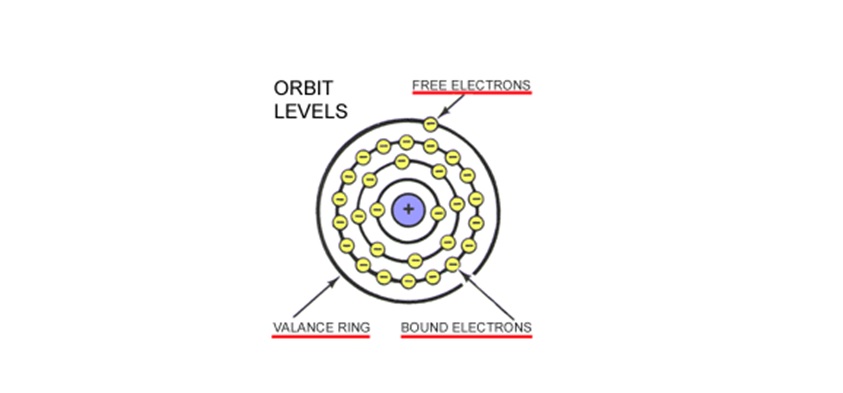
Electrons rotate around the atom at different orbits called Rings, Orbits, or Shells. BOUND ELECTRONS orbit the nucleus on the inner rings. Bound electrons have a strong magnetic attraction to the nucleus. FREE ELECTRONS orbit on the outermost ring which is known as the VALANCE RING.
Free Electrons:
Only the FREE ELECTRONS in the outermost shell (Valance Ring) are free to move from atom to atom. This movement is called ELECTRON FLOW. These FREE ELECTRONS are loosely held and can easily be moved to another atom or ion. Because of their distance from the nucleus, free electrons have a weak magnetic attraction. Since this attraction is not as strong to the nucleus as the bound electrons on the inner orbits, the free electrons move easily from atom to atom.
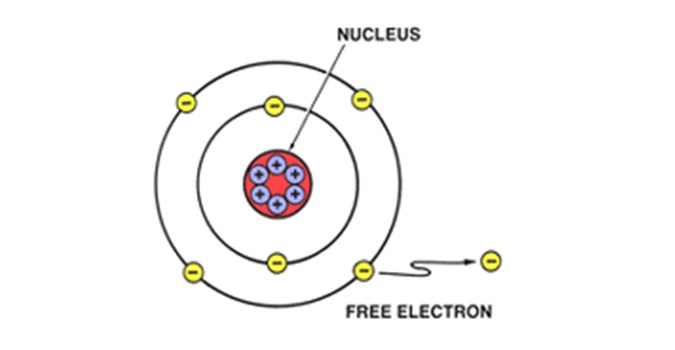
Free electrons are produced when some force disturbs the stable relationship of electrons and protons in an atom. This force, which "knocks" electrons out of orbit, can be produced in a number of ways, such as: by moving a conductor through a magnetic field; by friction, as when a glass rod is rubbed with silk; or by chemical action, as in a battery. The force "frees" the electrons from their atoms; these electrons are called free electrons. When an electric force is applied to a material such as copper wire, electrons in the outer orbits of the copper atoms are forced out of orbit and impelled along the wire.
The electrons that have been forced out of orbit are called free electrons. Electric Current depends on the movement of free electrons.
Electric Current:
The movement of free electrons along a wire is what we call electric current. it cannot exist where there are no free electrons.
Electrostatic Force:
Electrostatic force (also called Coulomb's law) is a force that operates between charges. It states that charges of the same type repel each other, while charges of opposite types are attracted together. Opposites attract, and likes repel.
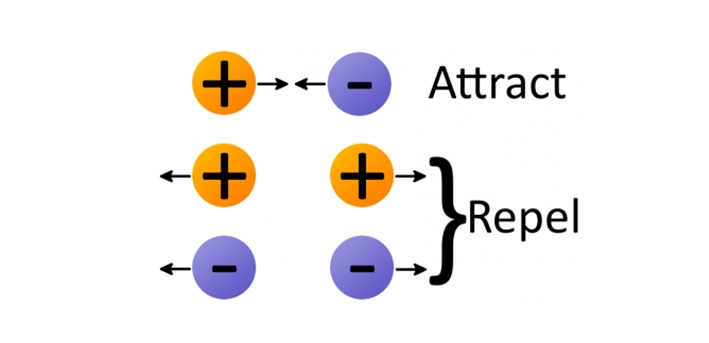
The amount of force acting on two charges depends on how far they are from each other. The closer two charges get, the greater the force (either pushing together, or pulling away) becomes.
Thanks to electrostatic force, electrons will push away other electrons and be attracted to protons. This force is part of the "glue" that holds atoms together, but it's also the tool we need to make electrons (and charges) flow!